中国组织工程研究 ›› 2016, Vol. 20 ›› Issue (30): 4515-4523.doi: 10.3969/j.issn.2095-4344.2016.30.016
• 生物材料综述 biomaterial review • 上一篇 下一篇
用于心血管医疗装置的聚合物材料表面构建与生物相容性评价:聚合物生物材料表面的内皮细胞组织工程化改性
陈宝林1,王东安2,3
- 1呼伦贝尔学院科技处,内蒙古自治区呼伦贝尔市 021008;2浙江大学高分子科学研究所,浙江省杭州市 310027;3 Department of Pharmaceutical Sciences, University of Tennessee Health Science Center, Memphis, Tennessee 38163, USA
-
收稿日期:2016-05-07出版日期:2016-07-15发布日期:2016-07-15 -
通讯作者:陈宝林,呼伦贝尔学院科技处,内蒙古自治区呼伦贝尔市 021008 -
作者简介:陈宝林,男,1960年生,河北省新城县人,汉族,1983年东北师范大学毕业,教授,主要从事组织工程材料(生物医用高分子材料)的制备及表征方面的研究。
Surface construction and biocompatibility of polymer materials as cardiovascular devices: modified tissue-engineered endothelial cells on the surface of polymeric biomaterials
Chen Bao-lin1, Wang Dong-an2, 3
- 1Bureau of Science & Technology Research, Hulunbuir College, Hulunbuir 021008, Inner Mongolia Autonomous Region, China
2Institute of Polymer Science, Zhejiang University, Hangzhou 310027, Zhejiang Province, China3Department of Pharmaceutical Sciences, University of Tennessee Health Science Center, Memphis, Tennessee 38163, USA
-
Received:2016-05-07Online:2016-07-15Published:2016-07-15 -
Contact:Chen Bao-lin, Bureau of Science & Technology Research, Hulunbuir College, Hulunbuir 021008, Inner Mongolia Autonomous Region, China -
About author:Chen Bao-lin, Professor, Bureau of Science & Technology Research, Hulunbuir College, Hulunbuir 021008, Inner Mongolia Autonomous Region, China
摘要:
文章快速阅读:
.jpg)
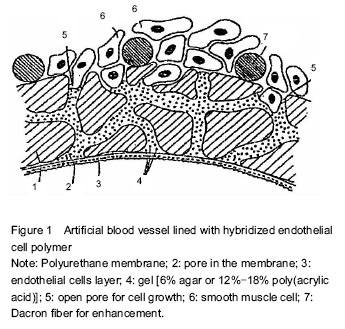
背景:用于心血管医疗的生物材料在血液接触性条件下必须具有抗血栓性、对抗生物降解性与抗感染性。 目的:综述用于心血管组织工程的新型植(介)入型聚合物材料(表面)研究进展,从聚合物生物材料表面的内皮细胞组织工程化改性方面考察各种相应改性表面的生物相容性、血液相容性和细胞相容性。 方法:第一作者计算机检索1963至2015年PubMed数据库及万方数据库。英文检索词为“Biocompatibility,Blood compatibility,Biomedical Materials,Biomedical polymer materials”,中文检索词为“生物相容性材料,血液相容性材料,生物医用材料,医用高分子材料”。排除与研究目的相关性差及内容陈旧、重复的文献,保留与生物医用高分子材料的血液相容性研究,进行归纳总结。通过对血管内皮细胞的功能、移植物表面的内皮细胞组织工程化、聚合物生物材料表面促细胞生长因子的固定方法、材料表面内皮化4方面的归纳分析,从聚合物生物材料表面的内皮细胞组织工程化改性方面考察了各种相应改性表面的生物相容性、血液相容性和细胞相容性。 结果与结论:共纳入71篇文献。研制用于心血管组织工程的新型植(介)入型聚合物材料(表面)关键在于对聚合物生物材料表面的内皮细胞组织工程化改性以及对其相应生物相容性与内皮细胞相容性的研究。通过对心血管医疗用聚合物生物材料的种类与应用及其心血管医疗器件和可植入性软组织替代物的深入研究可以发现材料表面与本体的差异则将体现在从表面向本体延伸的很多层分子上,而表面能和分子运动性这2种主要因素决定了其包括本体/表面差异及表面相分离在内的本体/表面行为。如果考虑到对本体-表面的组成差异的理解,则还必须追加另以附加决定因素,即各组分的结晶行为。
ORCID: 0000-0001-8283-783X(Chen Bao-lin)
中图分类号:
引用本文
陈宝林,王东安. 用于心血管医疗装置的聚合物材料表面构建与生物相容性评价:聚合物生物材料表面的内皮细胞组织工程化改性[J]. 中国组织工程研究, 2016, 20(30): 4515-4523.
Chen Bao-lin, Wang Dong-an. Surface construction and biocompatibility of polymer materials as cardiovascular devices: modified tissue-engineered endothelial cells on the surface of polymeric biomaterials[J]. Chinese Journal of Tissue Engineering Research, 2016, 20(30): 4515-4523.
| [1] Chen BL, Wang DA. Phase III study on surface construction and biocompatibility of polymer materials as cardiovascular devices: coagulant and anti-coagulant surface modification. Zhongguo Zuzhi Gongcheng Yanjiu. 2015;19(8):1277-1283. [2] Chen BL, Wang DA. Surface construction and biocompatibility of polymeric used for cardiovascular medical device. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18(21):3412-3419. [3] Chen BL, Wang DA. Surface construction and biocompatibility of polymeric used for cardiovascular medical device. Zhongguo Zuzhi Gongcheng Yanjiu. 2013;17(34):6183-6292. [4] Chen BL, Wang DA. Hemocompatibility of biomedical polymeric materials-design of anticoagulatent materials. Zhongguo Zuzhi Gongcheng Yanjiu. 2012;16(34): 6393-6396. [5] Yang ZM. Basic and Clinical Rusearch on Engineering. Chengdu: Sichuan Science and Technology Press. 2000: 239-247. [6] Ito RK, Rosenblatt MS, Contreras MA, et al. Monitoring platelet interactions with prosthetic graft implants in a canine model. ASAIO Trans. 1990;36(3): M175-M178. [7] McCollum CN, Kester RC, Rajah SM, et al. Arterial graft maturation: the duration of thrombotic activity in Dacron aortobifemoral grafts measured by platelet and fibrinogen kinetics. Br J Surg. 1981;68(1):61-64. [8] Gamble JR, Harlan JM, Klebanoff SJ, et al. Stimulation of the adherence of neutrophils to umbilical vein endothelium by human recombinant tumor necrosis factor. Proc Natl Acad Sci U S A. 1985;82(24):8667-8671. [9] Cameron BL, Tsuchida H, Connall TP, et al. High porosity PTFE improves endothelialization of arterial grafts without increasing early thrombogenicity. J Cardiovasc Surg (Torino). 1993;34(4):281-285. [10] Clowes AW, Kohler T. Graft endothelialization: the role of angiogenic mechanisms. J Vasc Surg. 1991;13(5):734-746. [11] Greisler HP, Ellinger J, Schwarcz TH, et al. Arterial regeneration over polydioxanone prostheses in the rabbit. Arch Surg. 1987;122(6):715-721. [12] Abbott WM, Cambria RP. Control of physical characteristics (elasticity and compliance) of vascular grafts. In: Stanley JC et al. (eds) Biologic and synthetic vascular prostheses, New York: Grüne and Stratton. 1982:189-220. [13] Hasson JE, Megerman J, Abbott WM, et al. Increased compliance near vascular anastomoses. J Vasc Surg. 1985;2(3):419-423. [14] Veith FJ, Gupta SK, Ascer E, et al. Six-year prospective multicenter randomized comparison of autologous saphenous vein and expanded polytetrafluoroethylene grafts in infrainguinal arterial reconstructions. J Vasc Surg. 1986;3(1):104-114. [15] Eberhart RC, Munro MS, Williams GB, et al. Albumin adsorption and retention on c18-alkyl-derivatized polyurethane vascular grafts. Artificial Organs. 1987; 11(5):375-382. [16] Tsai CC, Huo HH, Kulkarni P, et al. Biocompatible coatings with high albumin affinity. ASAIO Trans. 1990; 36(3):M307-M310. [17] Rumisek JD, Wade CE, Brooks DE, et al. Heat-denatured albumin-coated Dacron vascular grafts: Physical characteristics and in vivo performance. J Vasc Surg. 1986;4(2):136-143. [18] Kottke-Marchant K1, Anderson JM, Umemura Y, et al. Effect of albumin coating on the in vitro blood compatibility of Dacron® arterial prostheses. Biomaterials. 1989;10(3):147-155. [19] Goëau-Brissonnière L, Mercier F, Nicolas M, et al. Treatment of vascular graft infection by in situ replacement with a rifampin-bonded gelatin-sealed dacron graft. J Vasc Surg. 1994;19(4):739-741. [20] Graham LM, Burkel WE, Ford JW, et al. Expanded polytetrafluoroethylene vascular prostheses seeded with enzymatically derived and cultured canine endothelial cells. Surgery. 1982;91(5):550-559. [21] Jarrell BE, Williams SK. Microvessel derived endothelial cell isolation, adherence, and monolayer formation for vascular grafts. J Vasc Surg. 1991; 13(5):733-734. [22] Kempczinski RF, Rosenman JE, Pearce WH, et al. Endothelial cell seeding of a new PTFE vascular prosthesis, J Vasc Surg. 1985;2(3):424-429. [23] Wakefield TW, Lindblad B, Graham LM, et al. Nuclide imaging of vascular graft-platelet interactions: comparison of indium excess and technetium subtraction techniques. J Surg Res. 1986;40(4):388-394. [24] Graham LM, Fox PL. Growth factor production following prosthetic graft implantation, J Vasc Surg. 1991;13(5): 742-744. [25] Jensen N, Lindblad B, Bergovist D. Endothelial cell seeded dacron aortobifurcated grafts: platelet deposition and long-term follow-up. J Cardiovasc Surg (Torino). 1994;35(5):425-429. [26] Zilla P, Deutsch M, Meinhart J, et al. Clinical in vitro endothelialization of femoropopliteal bypass grafts: an actuarial follow-up over three years. J Vasc Surg. 1994; 19(3):540-548. [27] Magometschnigg H, Kadletz M, Vodrazka M, et al. Prospective clinical study with in vitro endothelial cell lining of expanded polytetrafluoroethylene grafts in crural repeat reconstruction. J Vasc Surg. 1992;15(3): 527-535. [28] Pasic M, Müller-Glauser W, Von Segesser L, et al. Superior late patency of small-diameter Dacron grafts seeded with omental microvascular cells: an experimental study. Ann Thor Surg. 1994;58(3): 677-684. [29] Greisler HP, Klosak J, Dennis JW, et al. Endothelial cell growth factor attachment to biomaterials. ASAIO Trans. 1986;32(1):346-349. [30] Gray JL, Kang SS, Zenni GC, et al. FGF-1 Affixation Stimulates ePTFE Endothelialization without Intimal Hyperplasia. J Surg Res. 1994;57(2):596-612. [31] Reilly CF, Kindy MS, Brown KE, et al. Heparin prevents vascular smooth muscle cell progression through the G1 phase of the cell cycle. J Biol Chem. 1989;264(12): 6990-6995. [32] Au YP, Kenagy RD, Clowes MM, et al. Mechanisms of inhibition by heparin of vascular smooth muscle cell proliferation and migration. Hamostasis. 1993;23(1): 177-182. [33] Kang SS, Gosselin C, Ren DW, et al. Selective stimulation of endothelial cell proliferation with inhibition of smooth muscle cell proliferation by fibroblast growth factor-1 plus heparin delivered from fibrin glue suspensions. Surgery. 1995;118(2):280-287. [34] Gosselin C, Ren DW, Ellinger J, et al. In vivo platelet deposition on polytetrafluoroethylene coated with fibrin glue containing fibroblast growth factor 1 and heparin in a canine model. Am J Surg. 1995;170(2):126-130. [35] Greisler HP, Schwarcz TH, Ellinger J, et al. Dacron inhibition of arterial regenerative activities. J Vasc Surg. 1986;3(5):747-756. [36] Greisle HP, Dennis JW, Endean ED, et al. Derivation of neointima in vascular grafts. Circulation. 1988;78(3 Pt 2):6-12. [37] Cifonelli JA. Relation of chemical of heparin to its anticoagulant activity. Adv Exp Med Biol. 1975;52:95-103. [38] Castillo EJ, Koenig JL, Anderson JM, et al. Protein adsorption on hydrogels. II. Reversible and irreversible interactions between lysozyme and soft contact lens surfaces. Biomaterials. 1985;6(5):338-345. [39] Drumheller PD, Hubbell JA. Surface immobilization of adhesion ligands for investigations of cell/substrate interactions. CRC and IEEE Press. 1995:1583-1596. [40] Beer JH, Springer KT, Coller BS, et al. Immobilized Arg-Gly-Asp (RGD) peptides of varying lengths as structural probes of the platelet glycoprotein IIb/IIIa receptor. Blood. 1992;79(1):117-128. [41] Silver JH, Hergenrother RW, Lin JC, et al. Surface and blood-contacting properties of alkylsiloxane monolayers supported on silicone rubber. J Biomed Mater Res. 1995; 29(4):535-548. [42] Wang CC, Tsai H, Shih HH, et al. Synthesis and characterization of poly(ethylene glycol) derivatives. J Poly Sci Poly Chem Ed. 1984;22(2):341-352. [43] Sawhney AS, Hubbell JA. Poly (ethylene oxide)-graft-poly(L-lysine) copolymers to enhance the biocompatibility of poly(L-lysine)-alginate microcapsule membranes. Biomaterias. 1992;13(12):863-870. [44] Kobayashi H, Ikada Y. Covalent immobilization of proteins on to the surface of poly(vinyl alcohol) hydrogel. Biomaterials. 1991;12(8):747-751. [45] Bannwarth W, Schmidt D, Stallard RL, et al. Bathophenanthroline-ruthenium(II) complexes as non-radioactive labels for oligonucleotides which can be measured by time-resolved fluorescence techniques. Helvetica Chimica Acta. 1988;71(8):2085-2099. [46] Liu SQ, Ito Y, Imanishi Y. Cell growth on immobilized cell growth factor. 9. Covalent immobilization of insulin, transferrin, and collagen to enhance growth of bovine endothelial cells. J Biomed Mater Res. 1993;27(7): 909-915. [47] Streeter HB, Rees DA. Fibroblast adhesion to RGDS shows novel features compared with fibronectin. J Cell Biol. 1987;105(1):507-515. [48] Werb Z, Tremble PM, Behrendtsen O, et al. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989;(2):109:877-889. [49] Jagendorf AT, Patchornik A, Sela M. Use of antibody bound to modified cellulose as an immunospecific adsorbent of antigens. Biochim Biophys Acta. 1963; 78(3):516-527. [50] Tseng YC, Park K. Synthesis of photoreactive poly(ethylene glycol) and its application to the prevention of surface-induced platelet activation. J Biomed Mater Res. 1992;26:373-391. [51] Yan MD, Cai SX, Wybourne MN, et al. Photochemical functionalization of polymer surfaces and the production of biomolecule-carrying micrometer-scale structures by deep-UV lithography using 4-substituted perfluorophenyl azides. J Am Chem Soc. 1993;115(2): 814-816. [52] Guire, Patrick E. Biocompatible device with covalently bonded biocompatible agent, United States Patent 5263992. 1993. [53] Yoshiaki S, Masahiro K, Masaya I. Surface analysis of antithrombogenic ion-implanted silicon rubber. Nucl Instrum Methods Phys Res B. 1991;59-60(Part 2): 1300-1303. [54] Steele JG, Johnson G, Norris WD, et al. Adhesion and growth of cultured human endothelial cells on perfluorosulphonate: pole of vitronectin and fibponectin in cell attachment. Biomaterials. 1991;12(6):531-539. [55] Klein-Soyer C, Hemmendinger S, Cazenave JP. Culture of human vascular endothelial cells on a positively charged polystyrene surface, primaria: comparison with fibronectin-coated tissue culture grade polystyrene. Biomateriala. 1989;10(2):85-90. [56] Rémy M, Bordenave L, Bareille R. Endothelial cell compatibility testing of various prosthetic surfaces. J Mater Sci. 1994;5(11):808-812. [57] Xu L, Jing ZP. Artificial vascular graft endothelialization of natural. Shanghai Shengwu Yixue Gongcheng Zazhi. 1997;18(2):31-32. [58] Jing ZP, Xu L. Experimental study of emdothelialization on domestic silk dacron graft. Shanghai Shengwu Yixue Gongcheng Zazhi. 1998;19(1):19-20. [59] Marko T, Peter B. Vascular graft wall. Eur Paitent. EP0248247, 1987. [60] Chen BL, Wang DA. Preparation and mechanism of anticoagulatent biomedical polymer materials with blood compatibility. Zhongguo Zuzhi Gongcheng Yanjiu. 2011; 15(29):5507-5510. [61] Chen BL, Wang DA, Feng LX. Investigation on methods of surface modification of tissue engineering materials: polymer surface group transformation and bioactive molecule immobilization. Zhongguo Zuzhi Gongcheng Yanjiu. 2010;14(3):552-554. [62] Chen BL, Wang DA, Feng LX. Surface modification of tissue-engineered materials plasma and grafting modification. Zhongguo Zuzhi Gongcheng Yanjiu. 2009; 13(3):587-590. [63] Chen BL, Wang DA, Feng LX. Application of polymer biomaterials in the tissue engineering. Zhongguo Zuzhi Gongcheng Yanjiu. 2008;12(6):1189-1192. [64] Chen BL, Wang DA, Feng LX. Polymer porous membrane prepared using thermally induced phase separation. Zhongguo Zuzhi Gongcheng Yanjiu. 2007; 11(40):8217-8219. [65] Chen BL, Wang DA, Feng LX. Topology of tissue engineered material surface for cell compatibility. Zhongguo Zuzhi Gongcheng Yanjiu. 2007;11(18): 3653-3656. [66] Chen BL, Wang DA, Feng LX. Effects of physical-chemical properties of tissue engineered material surface on cell compatibility. Zhongguo Zuzhi Gongcheng Yanjiu. 2007;11(1):197-200. [67] Chen BL, Wang DA, Feng LX. Cytological effect of tissue engineering materials with cell compatibility. Zhongguo Linhchunag Kangfu. 2006;10(45):225-227. [68] Chen BL, Wang DA, Feng LX, et al. The application of biomedical tissue engineering and the polymer tissue engineering material. Gaoshi Like Xuekan. 2007;27(1): 24-26. [69] Chen BL, Wang DA, Feng LX, et al. Study on the blood compatibility of biomedical ploymer materials-project of antithromboeicity materials. Suihua Xueyuan Xuebao. 2007;27(1):186-188. [70] Chen BL, Wang DA, Feng LX, et al. Study on surfaces modify of the tissue engineering materials and application in the tissue engineering. Hulunbeier Xueyuan Xuebao. 2007;15(1):52-54. [71] Chen BL, Wang DA, Feng LX, et al. Study on the tissue compatibility of biomedical ploymer materials-project of tissue-compatibility materials. Hulunbeier Xueyuan Xuebao. 2006;14(6):34-36. |
| [1] | 鲍玉成,张文龙,王 勇,于美丽,杨雪纯,申 靖. 聚乳酸羟基乙酸-环丝氨酸缓释微球植入剂的制备及其体外释药性能[J]. 中国组织工程研究, 2018, 22(6): 971-976. |
| [2] | 刘 霞,王文娟,吴雪梅,谢 红. 麻醉诱导期梯度效应室靶控输注丙泊酚应用对老年患者围术期血流动力学和苏醒期的影响[J]. 中国组织工程研究, 2018, 22(30): 4835-4840. |
| [3] | 刘 培,胡振生,马 玲,王换换,李 栋. 脂肪间充质干细胞条件培养基制备温敏凝胶外用治疗皮肤烫伤[J]. 中国组织工程研究, 2017, 21(30): 4852-4859. |
| [4] | 黄文良,叶 鹏,莫 刚,田仁元,马立坤,阮世强,徐 林,邓 江. 制备缓释骨形态发生蛋白2的丝素蛋白/壳聚糖/纳米羟基磷灰石生物支架[J]. 中国组织工程研究, 2017, 21(22): 3488-3493. |
| [5] | 张金哲,周群华,杨立群,张 巍,李建新,金 瑛,易东旭. 非生物降解型及生物降解型长效皮下埋植避孕剂的理论研究与应用进展[J]. 中国组织工程研究, 2017, 21(22): 3595-3601. |
| [6] | 孔 军,祝永强. 多孔磷酸钙颗粒对维生素C的负载及其可控释放[J]. 中国组织工程研究, 2017, 21(2): 273-279. |
| [7] | 王 勇,万永鲜,张喜海,叶俊武,卓乃强. 载万古霉素-羟基磷灰石涂层髓内钉治疗长骨开放性骨折带菌性伤口感染模型研究[J]. 中国组织工程研究, 2017, 21(14): 2163-2169. |
| [8] | 李文甜,涂计,高飞,刘国辉,邵增务,石磊,张祥林,熊蠡茗. 凝胶敷料用于骨外露创面的修复:创新与发展[J]. 中国组织工程研究, 2017, 21(10): 1617-1622. |
| [9] | 王骞,耿广起,丛晓明,刘海涛,施建党,王自立,马文鑫,孙宇航. 载三联抗痨药硫酸钙/聚氨基酸缓释材料在兔脊柱结核模型体内的缓释性能[J]. 中国组织工程研究, 2017, 21(10): 1520-1526. |
| [10] | 孙治邦,周义钦,陈 松,吴海山. 人β-防御素3/聚乳酸-羟基乙酸缓释微球制备及体外释药性能[J]. 中国组织工程研究, 2017, 21(10): 1514-1519. |
| [11] | 高昊辰,汪 沛,曹志中,葛葵葵,汪益涵,陆 敏. 载米诺环素纳米羟基磷灰石/壳聚糖复合体的体外释放及抑菌性[J]. 中国组织工程研究, 2016, 20(8): 1118-1125. |
| [12] | 邬 瑾. 非洛地平缓释片在中国高血压患者治疗中的应用[J]. 中国组织工程研究, 2016, 20(47): 7127-7132. |
| [13] | 卢晓郎,郑亦静,程 涛,叶 超,洪建军. 骨形态发生蛋白缓释微球结合富血小板凝胶对骨髓基质干细胞增殖分化的影响[J]. 中国组织工程研究, 2016, 20(47): 7057-7063. |
| [14] | 陈 瑶. 高分子药用控释及缓释载体材料特点及在高血压治疗中的应用[J]. 中国组织工程研究, 2016, 20(43): 6530-6536. |
| [15] | 王非凡,宋云嘉,吴文孟,吕武龙,李 莺,李长义. 纯钛表面淫羊藿苷/TiO2纳米管复合涂层制备与药物早期释放的分析[J]. 中国组织工程研究, 2016, 20(43): 6416-6423. |
Data sources
Totally 193 articles were retrieved initially, including 44 Chinese literature and 149 English literature. Among them, 122 articles were excluded because of poor correlation with the research purpose and old or repetitive content. Finally, 71 articles were included in result analysis[1-71], addressing the host response, clinical applications, new techniques and legal issues of the existing biomaterials[1-11]; the functions of vascular endothelial cells[12-27]; the tissue-engineered endothelial cells on the surface of grafts[11, 28-36]; the fixation of cell growth- promoting factor on the surface of polymeric biomaterials[37-53]; the endothelialization of the material surface[54-71].
血管内皮细胞的功能、移植物表面的内皮细胞组织工程化、聚合物生物材料表面促细胞生长因子的固定、材料表面内皮化影响用于心血管组织工程的新型植入型聚合物材料的治疗效果。通常对材料进行表面分子设计,改善表面的亲硫水性、引入带电基团、负载生物活性物质等,以尽量减轻血栓的形成来提高材料的血液相容性,减少患者的不良反应。
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
.jpg)